What are the three types of charge on the particle?
What are 3 particle charges
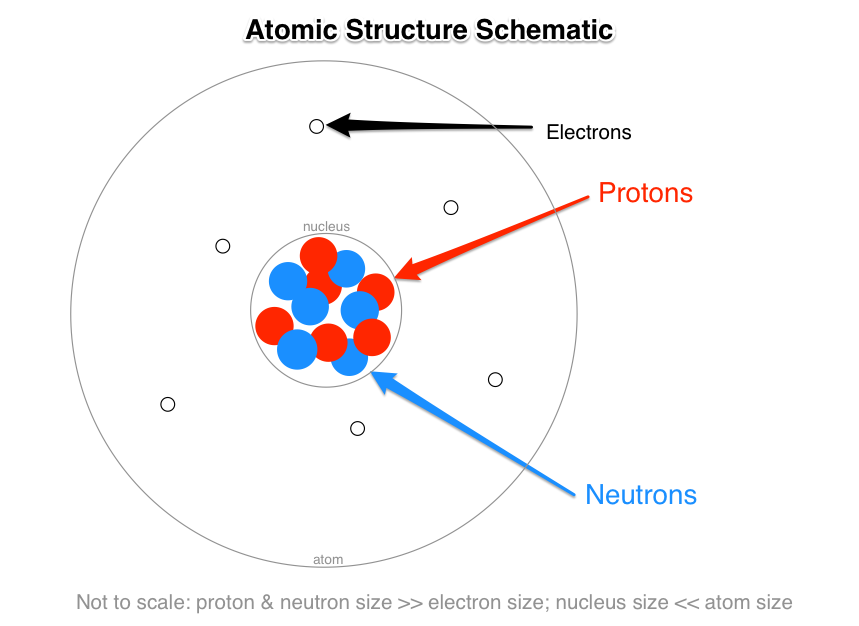
There are three subatomic particles: protons, neutrons and electrons. Two of the subatomic particles have electrical charges: protons have a positive charge while electrons have a negative charge. Neutrons, on the other hand, don't have a charge.
What are the 3 types of charges
There are three types of electric charges – positive, negative and neutral.
What are the three 3 charges of an atom
Our current model of the atom can be broken down into three constituents parts – protons, neutron, and electrons. Each of these parts has an associated charge, with protons carrying a positive charge, electrons having a negative charge, and neutrons possessing no net charge.
What are the different types of charged particles
In matter, these charged particles or subatomic particles are negatively charged electrons, positively charged protons, and neutral neutrons.
Where are the 3 subatomic particles charges
All the positive charge of an atom is contained in the nucleus, and originates from the protons. Neutrons are neutrally-charged. Electrons, which are negatively-charged, are located outside of the nucleus.
What are the four charged particles
Four charge particles α, proton, deuteron and Li+++ are projected in a uniform magnetic field perpendicular to their velocity.
What are the 3 ways to get charge
Methods of chargingCharging by friction.Charging by conduction.Charging by induction.
What are the 3 basic properties of charges
Some other properties of electric charges are –Charge is a scalar quantity.Charge transfer from one body to another which means they are movable.Like charges repel each other and unlike charges attract each other.Charge is always linked with mass.
Which ions have a 3 charge
Group III A (13) metals form cations with +3 charge. Please note that the first element in this group, boron (B) is a non-‐metal and typically doesn't form a cation.
What are the types of charges in an atom
Electric charge comes in two main types: positive and negative charges. Positive charges are associated with protons, which are subatomic particles residing in the nucleus of an atom. They are represented by the symbol “+”.
What are the charges of protons neutrons and electrons
The charge on the proton is positive, whereas the charge on the electron is negative. As opposite charges attract, it is partly the positive charge of the protons that keeps the electrons in “orbit” around the nucleus. Neutrons carry no charge.
What are the 3 subatomic particles of an atom quizlet
Protons, neutrons, and electrons are the three main subatomic particles found in an atom. Protons have a positive (+) charge.
What is the charge on a particle
A charged particle, also called an ion, is an atom with a positive or negative charge. This happens whenever something called an ionic bond forms. Two particles that have different numbers of electrons (the smallest particle in an atom which is negative) start reacting to each other.
What are three ways of charging an object
In order to charge an object, one has to alter the charge balance of positive and negative charges. There are three ways to do it: friction, conduction and induction.
How many types of charges are there
two
Electric charges are of two general types: positive and negative. Two objects that have an excess of one type of charge exert a force of repulsion on each other when relatively close together.
Are there 3 basic rules of electric charge
Like charges attract, while unlike charges repel each other. Like charges repel, while unlike charges attract each other. Tapes having positive charge repel, while tapes having negative charge attract each other.
What ions have a charge
Ions with a positive charge are called cations. Ions with a negative charge are called anions. Many normal substances exist in the body as ions. Common examples include sodium, potassium, calcium, chloride, and bicarbonate.
Which anion has 3 units of negative charge
Thus, name of ion having three units of negative charge is nitride ion .
What are the 3 parts of an atom and their charge where is each located
Nucleons include protons and neutrons. All the positive charge of an atom is contained in the nucleus, and originates from the protons. Neutrons are neutrally-charged. Electrons, which are negatively-charged, are located outside of the nucleus.
What are the 3 main components of an atom and the charges they hold
The three basic parts of an atom are electrons, protons, and neutrons. Protons are positively charged, electrons are negatively charged and neutrons are neutral.
