What is the highest IMF?
Which element has the highest IMF
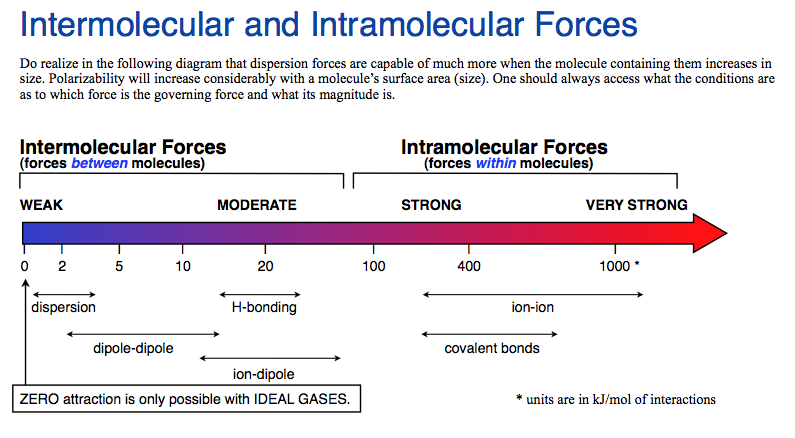
The strongest intermolecular force is hydrogen bonding, which is a particular subset of dipole-dipole interactions that occur when a hydrogen is in close proximity (bound to) a highly electronegative element (namely oxygen, nitrogen, or fluorine).
Cached
Which bond has the strongest IMF
Hydrogen bonds
Hydrogen bonds are a special case of dipole-dipole interactions. H-bonds are the strongest intermolecular force. (They are worth ca. 5 kJ•mol-1).
What state of matter has the strongest IMF
Solid
Solid usually have the strongest intermolecular forces when compared to liquids and gases. In solids, the particles are closely packed and this is why they are incompressible and have high density.
What phase has the strongest IMF
solid state
For any given substance, intermolecular forces will be greatest in the solid state and weakest in the gas state.
Do polar molecules have higher IMF
The polar substance always has the higher boiling point, indicating greater attractive forces between separate molecules, that is, larger intermolecular forces.
Does water have the highest IMF
Water possesses the strongest of intermolecular forces, hydrogen bonding. Remember that hydrogen bonding is a special case and strongest of dipole-dipole attraction.
What is the weakest IMF
the London dispersion forces
The weakest intermolecular force is the London dispersion forces. London dispersion force: London dispersion forces are temporary attractive forces that develop temporary dipole and hence they are also known as induced- dipole-induced-dipole.
What are the 3 strongest bonds
Therefore, the order from strongest to weakest bond is Ionic bond > Covalent bond > Hydrogen bond > Vander Waals interaction.
What is the strongest IMF to the weakest
The order of strength in the decreasing order is: ion-dipole, hydrogen bonds, dipole-dipole, and Vander Waals Forces.
What phase of matter has the weakest IMF
gaseous state
The state of matter with the least intermolecular forces of attraction is the gaseous state.
Does higher polarity mean stronger IMF
As polarity increases, the IMF increase and the mp and bp increase. This is because the stronger the IMF the harder it is to pull these molecules apart so a higher temperature is needed for melting and boiling.
Do larger molecules have higher IMF
The reason is that longer molecules have more places where they can be attracted to other molecules. This is the reason why pentane (longer chain molecule) experiences stronger intermolecular forces of attraction than methane.
Does water or oil have a higher IMF
Since hydrogen bonds are the strongest intermolecular force, the molecules of water are going to have higher surface tension than the molecules of the mineral oil.
Do solids or liquids have higher IMF
Solids have the strongest intermolecular force of attraction. In liquids, it is less than solid but more than gases and in gases, it is very weak.
What is the 2nd strongest IMF
The order of strength in the decreasing order is: ion-dipole, hydrogen bonds, dipole-dipole, and Vander Waals Forces.
What is the strongest IMF in water
hydrogen bonds
Water possesses dipole-induced dipole and London dispersion forces because it has hydrogen bonds. The greatest force is hydrogen bonds, although other forms of intermolecular attraction are also present.
What is the order of bonds from strongest to weakest
Therefore, the order from strongest to weakest bond is Ionic bond > Covalent bond > Hydrogen bond > Vander Waals interaction.
What is the strongest intermolecular interaction
Dipole-dipole interactions are the strongest intermolecular force of attraction.
Which IMF is the strongest and which is the weakest
Intermolecular forces In the order of weakest to strongest:dispersion force.Dipole-dipole force.Hydrogen bond.Ion-dipole force.
What is the strongest IMF solid
Dipole-dipole interactions are the strongest intermolecular force of attraction.
