Which net charge could be found on an object?
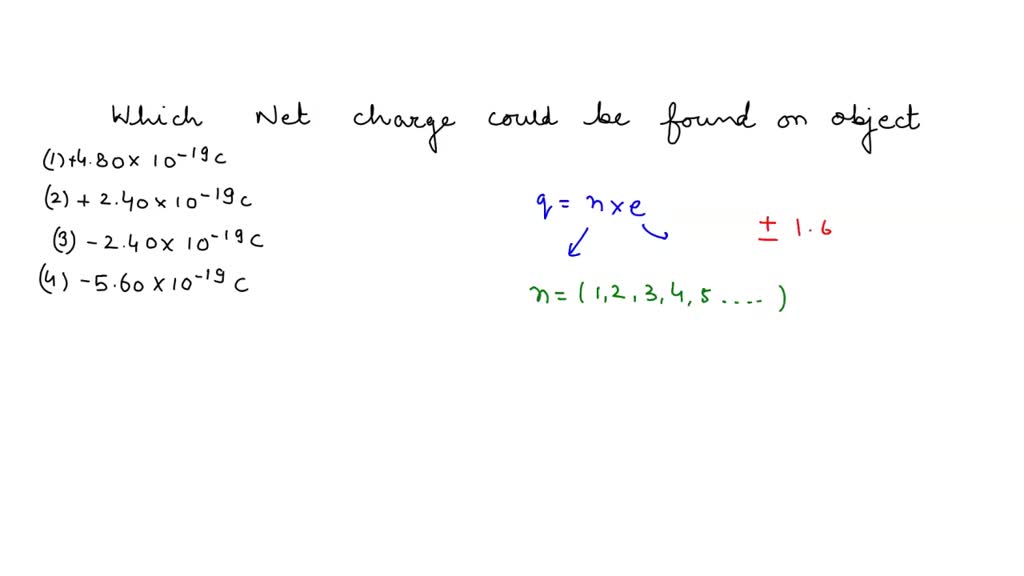
How is net charge found
To calculate a net charge, multiply the number of electrons gained or lost by the charge of an electron.
Cached
What are the 3 types of charges
There are three types of electric charges – positive, negative and neutral.
Is a electron positive or negative
negatively charged
An electron is a negatively charged subatomic particle that can be either bound to an atom or free (not bound).
Where is the net charge of an atom
An atom consists of a positively charged nucleus, surrounded by one or more negatively charged particles called electrons. The positive charges equal the negative charges, so the atom has no overall charge; it is electrically neutral.
Is net charge always 0
The net charge on the formula of a compound is always zero. This ensures that the compound is electrically neutral.
What is called net charge
An ion is an atom (or group of atoms) that has lost one or more electrons, giving it a net positive charge (cation), or that has gained one or more electrons, giving it a net negative charge (anion).
What are the types of charges
Electric charges are of two general types: positive and negative.
What are examples of charge
Charge Examples in Science
By convention, electrons have a charge of -1 while protons have a charge of +1. Another way of indicating charge is for an electron to have a charge of e and a proton to have a charge of +e. Quarks possess what is known as color charge.
Is a proton or electron negative
Electrons are a type of subatomic particle with a negative charge. Protons are a type of subatomic particle with a positive charge. Protons are bound together in an atom's nucleus as a result of the strong nuclear force.
What is a negative charge called
Ions with a negative charge are called anions.
What is the net charge of an electron
From the definition of the ampere, the electron itself has a negative charge of 1.602176634 × 10−19 coulomb.
Is the atomic number the net charge
And 10 electrons. Go ahead and determine the atomic number mass number net electric charge and the identity of the element. So the atomic number is the same as the number of protons it's nine the mass
Is net charge always negative
If an object has more protons than electrons, then the net charge on the object is positive. If there are more electrons than protons, then the net charge on the object is negative. If there are equal numbers of protons and electrons, then the object is electrically neutral.
What is a 0 net charge
If an atom has an equal number of protons and electrons, its net charge is 0.
What is an example of a net charge
"Net" means the total after taking account both positive and negative charges. So, for example, if something contains 321 positive charges and 319 negative charges, the net charge is 321 – 319 = +2. If it contains 37 positive charges and 42 negative charges, the net charge is 37 – 42 = -5.
What is meant by net charge quizlet
The net charges are positive, negative, and neutral(there is an equal amount of protons and electrons). Net Charge. How protons and electrons balance each other out. for example, if an Oxygen atom has 8 protons and 9 electrons, it has a negative net charge of +1(there are more electrons than protons).
What is net charge
"Net" means the total after taking account both positive and negative charges. So, for example, if something contains 321 positive charges and 319 negative charges, the net charge is 321 – 319 = +2. If it contains 37 positive charges and 42 negative charges, the net charge is 37 – 42 = -5.
Is proton positive or negative
positively charged
On the atomic level, protons are positively charged and electrons are negatively charged.
Is an proton charge positive or negative
The proton's positive charge is equal and opposite to the negative charge on an electron, meaning a neutral atom has an equal number of protons and electrons.
What is an example of a negative charge
When a balloon is rubbed on hair, fur or wool, electrons are moved from the hair to the balloon, giving the balloon a negative charge.
